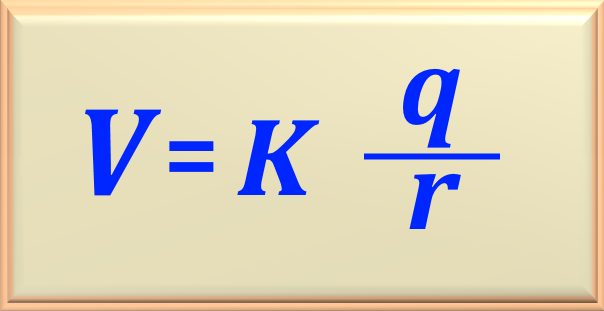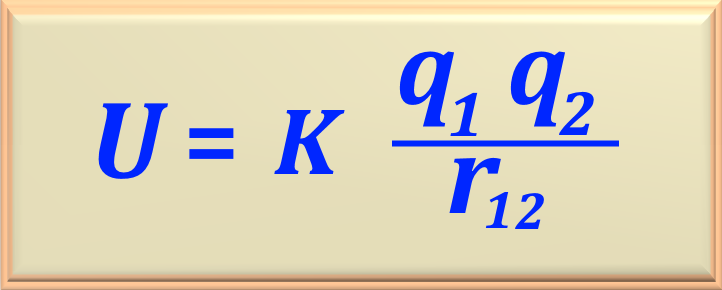Problem 72-1
Source: Example 23-2 - TIPLER, Paul - MOSCA, Gene -
Book: Física - Vol. 2 - 5ª Edição - Ed. LTC - 2006.
a) Calculate the electrical potential at a distance r = 0.529 x 10-10m of a proton
(This is the average distance between a proton and an electron in the hydrogen atom).
b) What is the potential electrical energy of the electron and proton at this
separation distance?
Solution of the Problem 72-1
The potential due to the proton can be calculated using eq. 72-09, reproduced below.
Then, replacing with the numerical values, we find:
Performing the calculation we find the value of the electrical potential, or
To calculate the potential energy between the two particles we can use eq. 72-11,
reproduced below.
Then, replacing with the numerical values, we find:
Performing the calculation we find the value of the potential energy, or
Dividing the value found by the particle charge, we obtain the energy value in eV, that is,
U = 27.20 eV. Note that using the eq. 72-07, since we know ΔV, let's
get the same result. Check it out !!!!
Note: If the electron were at rest, at this distance from the proton, at least
27.2 eV would be needed to remove it from the atom. However, as the electron revolves
around the proton, it has a kinetic energy equal to 13.6 eV. So, the total energy that
the electron has in the atom is 13.6 - 27.2 = -13.6 eV . Therefore, the minimum
energy required to remove the electron from the atom is 13.6 eV .
This energy is known as ionization energy.